
The National Statement on Ethical Conduct in Human Research (2025) outlines the ethical principles and standards for conducting research involving human participants. It is the responsibility of all researchers and research ethics offices to remain up to date with the current version of the National Statement when preparing research proposals, submitting applications for ethics review, and conducting ethics reviews.
In addition, researchers are responsible for ensuring their research complies with the ethical principles set out in the National Statement.
Researchers must examine the effect of their research on all participants — whether a person is a knowing participant or not — as well as any adverse effect the research may have on the lives of those who may be connected with, but not directly participating in, the research.
Expectations for Swinburne researchers conducting research involving humans:
- Be familiar with relevant Swinburne policies and procedures that concern human research activity.
- Be familiar with applicable sections and details in the National Statement and any applicable legislation or other guidelines.
- Plan for their research activity by including realistic timeframes as well as methods and outcomes that are academically, professionally and ethically sound.
- If applicable, use the appropriate form(s) and apply for prior ethical review from the designated ethical review body.
- Only commence human research activity after ethics clearance has been expressly issued and only do so in full compliance with the terms of the clearance.
- Apply for prior clearance for proposed amendments to current approved protocols.
- If there’s an emergency that requires research protocols to be amended quickly, such as to ensure the safety of those involved, notify the Swinburne University Human Research Ethics Committee and/or other relevant people as soon as possible.
- At a minimum, report annually and at the conclusion (or cessation) of any approved human research activity.
Further information
-
Human research requiring ethical review
Find out what research requires ethical review and what level of review is required, or whether your research is exempt from needing a review.
-
How to apply for ethical review
Find out more about submitting a human ethics application including the submission deadlines.
-
Monitoring, reporting and changes after approval
Find out how to request any modifications or changes to your approved application as well as the ongoing reporting requirements.
-
Frequently asked questions
Have questions about the ethical requirements surrounding human research? Find your answer here.
Ethics advisers
If you’re a Swinburne researcher, your first point of contact for seeking advice on human research ethics should be the relevant adviser.
Research Ethics Advisors (REAs) are members of Swinburne’s Human Research Ethics Sub-committees and are available to advise on research ethics queries from staff and students.. The role of REAs is not to write your ethics application, but to provide support by:
- advising on whether your research requires ethics review and what level of review is needed (ie. SUHREC or SHESC);
- highlighting discipline-specific ethical issues in research activities;
- assisting with the design of your research to ensure compliance with the National Statement on Ethical Conduct in Human Research;
- conducting a pre-review of draft ethics applications prior to submission (note: this is different to peer-review);
- assisting with response to committee feedback post-review;
- providing general advice on ethics processes at Swinburne
Please be respectful of the Advisors’ other commitments by allowing sufficient time for them to consider and respond to your queries.
For student projects, assistance from a Research Ethics Advisor should be done in conjunction with your supervisor.
The following information provides contact details for REAs and a description of their research areas.
Swinburne Australia
Swinburne Sarawak
| Faculty |
Research ethics adviser |
|---|---|
| Faculty of Engineering, Computing and Science |
Professor Bee Theng Lau |
| Associate Professor Hwang Siaw San |
|
| Associate Professor Viknesh Andiappan | |
| Dr Irine Runnie Henry Ginjom |
|
| School of Business, Faculty of Business, Design & Arts | Dr Leong Choi Meng |
| Dr Komathi Wasudawan |
Further resources and guidelines
- Consent in Human Research fact sheet [PDF 3.96MB]
- Recruitment in Human Research fact sheet [PDF 2.7MB]
- Program vs. Project Studies fact sheet [PDF 421KB]
- Guide to Informed Consent Instruments [DOCX 46KB]
- Guide to Informed Consent Instruments (Sarawak) [DOCX 41KB]
Swinburne policies:
The Swinburne University Human Research Ethics Committee (SUHREC) reviews human research proposals to ensure that they accord with the National Statement on Ethical Conduct in Human Research (2025) and are ethically acceptable before giving approval for the project’s commencement. Each SUHREC meeting is constituted and operated in accordance with the provisions of the National Statement.

SUHREC terms of reference
Current committee
| Membership category |
Member(s) |
|---|---|
| Chairperson |
Professor Timothy Marjoribanks |
| Lay people |
Ms Jen Lawrie-Smith Mr Iain Messer Ms Lesley Milburn Mr Lindsay Stodden |
| Professional care or counselling |
Ms Catherine Cross Dr Charmaine Gittleson Dr Julie Stevens Dr Karen Wayne |
| Rabbi Dr Benjamin Elton | |
| Rev. Peter Keeley | |
| Lawyer |
Dr Mitchell Adams Mr Paul Natoli Mr Dominic Brown |
| Researcher |
A/Prof. César Albarrán-Torres Dr Sharon Grant Professor Maja Nedeljkovic Professor Sonja Pedell A/Prof. Paul Scifleet A/Prof. Julian Vieceli Dr Stella Koritsas Dr Sean Carruthers Dr Ravi Iyer Associate Professor Ant Sowards Professor Suzi Hutchings |
Apply for committee membership
If you’re interested in becoming a member of the Swinburne University Human Research Ethics Committee (SUHREC), please download these two documents:
Apply for sub-committee membership
The SUHREC sub-committees are known as SHESC (Swinburne Human Ethics Sub-Committee). Consistent with the National Statement, the sub-committees review low-risk research involving people or their data or tissue.
If you’re a current Swinburne researcher and are interested in becoming a member of a SUHREC sub-committee, please contact resethics@swinburne.edu.au.
Swinburne researchers accessing, collecting, retaining, using or disclosing data pertaining to individuals will in some way be covered by various Commonwealth or Victorian legislation or guidelines on privacy of people or their information. The terms “personal information”, “health information” and “sensitive information” have particular definitions in the legislation and associated Principles.
Clear language and meaning is required regarding “identifiable”, “re-identifiable”, "potentially identifiable", “non-identifiable” or “de-identified” information or data. For example: “de-identified” can mean temporary removal of names and substitution with codes that permit later rematching of data.
Researchers should also take care with “anonymity” (literally no name) and “confidentiality” (the information collected or used may not use names but may still permit someone’s identity to be worked out). When planning their research, researchers should give due consideration both to the legal and ethical issues involved.
Some guidance is provided within Swinburne’s Privacy Guidelines and Standards. Further applicable legislation or standards depends on where the information is being collected or held — such as by a Commonwealth agency, a Victorian Public Sector Organisation, a Health Service Provider or a Private Sector body — and in what form.
Australian privacy law:
- Office of the Australian Information Commissioner website
- Guidelines approved under section 95 of the Privacy Act 1988 (March 2014)
- Guidelines under section 95A of the Privacy Act 1988 (March 2014)
Victorian privacy law:
For any research involving clinical trials or innovative therapy or intervention, see Chapter 3.1 of the National Statement on Ethical Conduct in Human Research (2025).
Is my research project a clinical trial?
In Australia, the NHMRC accepts the World Health Organisation's definition of a clinical trial as: "Any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes".1
Clinical trials study a range of interventions2 including (but not limited to):
- pharmaceutical interventions: e.g. experimental drugs, neutraceuticals, biological products, vaccines
- disease detection and treatment methods: new ways to detect and treat disease, diagnostic or screening tests
- therapeutic strategies: pyschotherapeutic and behavioural therapies
- medical or surgical procedures and devices
- health related service changes
- preventive care and educational interventions.
The NIH have developed a Clinical Trial decision tree3 that may assist in determining whether your study meets the definition of a clinical trial.
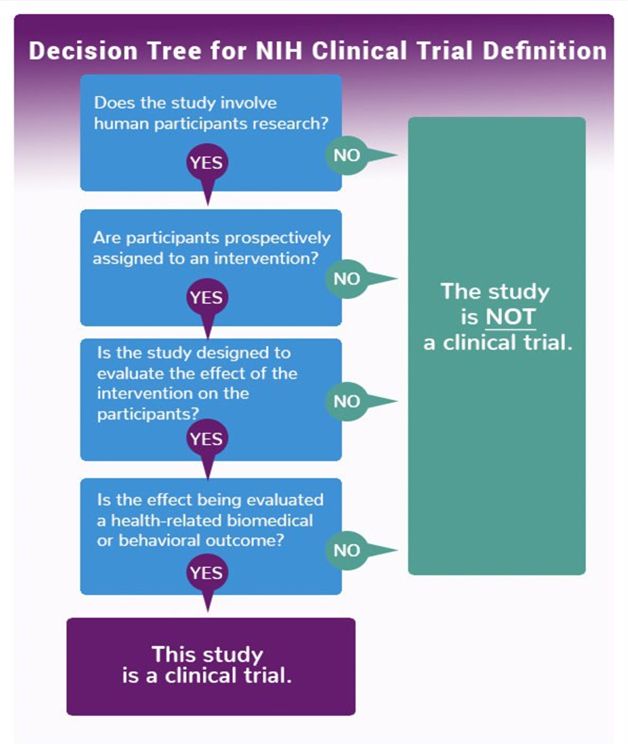
Sources:
1. World Health Organisation
2. Australian Clinical Trials
3. US National Institute of Health Clinical Trial Decision Tree
What is research governance?
Human research ethics approval and research governance approval are two essential aspects of a clinical trial that must both be approved before any new clinical trials can commence at Swinburne University of Technology. This a responsibility of the Chief Investigator (or their delegate) to complete. You do not need to wait for ethics approval before starting the research governance process.
Whilst the ethical review process ensures research proposals conform with ethical standards, the research governance process is a site-specific due diligence review to ensure compliance with relevant legislation, regulations, guidelines and codes.
The research governance process assesses whether appropriate resources/facilities, expertise, legal compliance, financial oversight, accountability and risk management have been considered and helps to identify and mitigate institutional risk.
The safety and protection of research participants is the fundamental basis of research governance. Everyone involved in a research project, including the research team, the institute where the research is taking place, the Sponsor of the research and governing bodies overseeing research are responsible and accountable for ensuring that research is conducted in a safe manner and to a high standard.
The research governance process starts early, in fact as soon as you have a proposal or idea it is encouraged to start thinking about and identifying areas of your project that will need to be addressed. These include, but are not limited to:
- adequate resources and facilities e.g. feasibility, evidence of researcher qualifications including appropriate training, authorisation to practice and experience, supervision of students, approval/s from facilities/supporting departments/centres
- financial assessment of proposed research including budget and funding review
- risk management e.g. insurance/indemnity, intellectual property arrangements
- applicable legal, regulatory, and jurisdictional requirements e.g research agreements/contracts, CTN (where applicable for drug/device trials), clinical trial registration, department of health approval for restricted drugs or substances.
The Research Office has a suite of templates and guidance, available on request, to assist researchers with their application for Research Governance authorisation. Please email resgovernance@swinburne.edu.au if your project requires Research Governance authorisation.
Clinical trials involving drugs or devices that require a CTN
Applications for Clinical Trial Notifications (CTN) to the Therapeutic Goods Administration (TGA) are to be submitted centrally via the Research Governance Lead, however researchers will be responsible for drafting the CTN.
- email resgovernance@swin.edu.au to request a user log in. Once the user (researcher) has been added by the ethics office, the researcher will receive a log in email separately from the TGA.
- the researcher drafts the application and submits a draft version to the Research Governance Lead for review and attaches the draft version in the ethics application in ERM.
- when the project has received HREC approval, and there are no imminent changes to the project, the researcher notifies the Research Governance Lead who will SUBMIT the CTN on behalf of the researcher.
- submitting a CTN raises an invoice which will be passed on to the researcher for payment.
- once the invoice has been paid, provide evidence (ie receipt) to the Research Governance Lead so that the submission can be finalised.
Clinical trials registration (Australia or international)
Swinburne researchers are obliged to register all clinical trials with a recognised clinical trials registry. Registration needs to occur prior to commencement of a trial. The leading registry in Australia is the Australian New Zealand Clinical Trials Registry (ANZCTR).
Additional resources for any research involving clinical trials or innovative therapy or intervention
- Chapter 3.1 of the National Statement on Ethical Conduct in Human Research (2025).
- World Medical Association Declaration of Helsinki — Ethical Principles for Medical Research Involving Human Subjects
- Therapeutic Goods Administration (TGA) website information on clinical trials, including the ICH Guideline for Good Clinical Practice and Australian Clinical Trial Handbook
- Australian Clinical Trials Website for useful information sponsored by the Australian Government and the National Health and Medical Research Council that includes information on 'Safety monitoring and reporting in clinical trials [PDF 523KB].
- Australian Code for the Responsible Conduct of Research 2018
- National Clinical Trials Governance Framework
- The Coordinating Office for Clinical Trial Research for Victorian State Government guidance - Clinical Trials and Research in Victoria
To undertake research in schools, researchers have to first establish what prior authority is required to approach or involve schools. In the case of Victorian Government schools, prior ‘in principle’ Departmental approval is required before principals can be approached to involve any staff or students. A similar arrangement of prior approval from the Director of the Catholic Education Office is required for Melbourne Catholic Diocesan schools.
Note: There are some exceptions to procedures for government and Catholic schools, such as when a student researcher is also a staff member of a particular school. However, the required proper procedure should be established early when planning to undertake research in schools.
In the case of independent schools, researchers can usually seek approval in the first instance from the head of school.
Further information:
Proposals for research involving Aboriginal and Torres Strait Islander Peoples should be developed in respectful consultation with leaders and/or members of the communities concerned and in consultation with the applicable guidelines:
- Health and other research: National Statement on Ethical Conduct in Human Research (2025).
- NHMRC health research: Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities: Guidelines for researchers and stakeholders (2018)
- Social and Cultural Research: AIATSIS Guidelines for Ethical Research in Australian Indigenous Studies
- Australia Council for the Arts: Indigenous Cultural Protocols for Producing Indigenous Music; Writing; Visual Arts, Media Arts; and Performing Arts (2007)
Please contact the Senior Research Ethics Coordinator on +61 3 9214 3845 or resethics@swinburne.edu.au as soon as possible if you are considering or proposing research involving Aboriginal and Torres Strait Islander Peoples (or other Indigenous Peoples) in case additional requirements have to be met. All proposals significantly involving Indigenous Australians require ethical review by SUHREC, with extra-committee confidential advice obtained as appropriate.
Proposals for research involving overseas Indigenous or First Nations Peoples should take note of applicable guidelines issued within the relevant jurisdiction.
Explore ethics and integrity topics
Contact us for more information
If you’re unsure about any aspects of human research or would like more information, please contact Research Ethics by emailing resethics@swinburne.edu.au.

